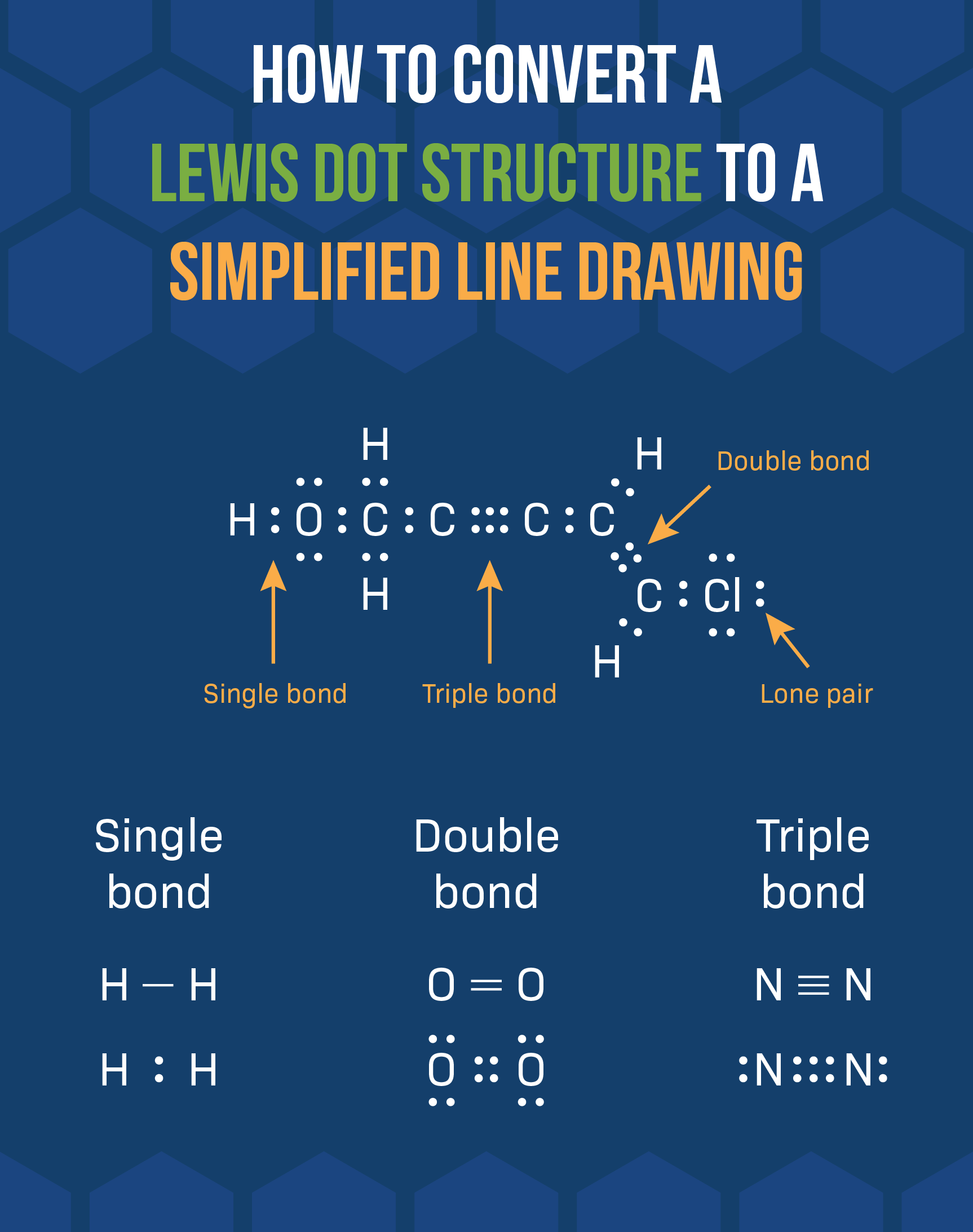
In chemistry, a Lewis dot structure is a diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. These structures are named after Gilbert N. Lewis, who introduced the concept in 1916. Drawing a Lewis dot structure is essential for understanding the chemical properties and reactivity of a molecule.
Creating a Lewis dot structure involves following a set of rules to determine the placement of atoms and electrons. The structure helps to visualize the arrangement of valence electrons and predict the geometry of a molecule. Understanding how to draw a Lewis dot structure is fundamental in the study of chemical bonding.
How do you draw a Lewis dot structure?
To draw a Lewis dot structure, start by counting the total number of valence electrons for all atoms in the molecule. Then, determine the central atom by looking for the least electronegative element. Next, connect the outer atoms to the central atom using single bonds. Distribute the remaining electrons as lone pairs to satisfy the octet rule for each atom. Adjust the structure as needed to minimize formal charges and achieve the most stable arrangement.
When drawing a Lewis dot structure, it is important to remember that hydrogen and helium follow the duet rule, requiring only two electrons to complete their outer shells. Elements in periods 3 and beyond can have expanded octets, meaning they can accommodate more than eight electrons in their valence shells. Additionally, resonance structures may be needed for molecules with delocalized electrons.
Once the Lewis dot structure is complete, analyze the arrangement of atoms and electrons to predict the molecular geometry. The shape of the molecule is determined by the number of bonding pairs and lone pairs around the central atom. Understanding the geometry of a molecule is crucial for predicting its physical and chemical properties.
In conclusion, drawing a Lewis dot structure is a fundamental skill in chemistry that helps to visualize the bonding and electron distribution in a molecule. By following the rules for assigning electrons and arranging atoms, one can create a clear representation of the structure. Understanding how to draw a Lewis dot structure is essential for predicting the reactivity and behavior of molecules in chemical reactions.